Last weekend 19 neurologists and researchers gathered in a hotel seminar room in The Netherlands to talk about building closer links across Europe.
Prof Leonard van den Berg, the current chair of the European ALS Consortium organised the workshop to work out how we can collaborate more effectively across Europe. Prof van den Berg is a neurologist specialising in MND with a busy and active research group, based in Utrecht in The Netherlands.
Ultimately we would like to have the infrastructure in place so that European researchers can react quickly to new opportunities in MND. For example, what would we want to do if another drug like lithium came along?
In 2009 a number of clinical trials to investigate whether lithium carbonate may be effective in people with MND were organised and funded on a national basis across Europe. (The UK clinical trial is still ongoing, although closed to recruitment). These trials represented a milestone in MND clinical research. Why? Because they were the first MND trials for many years that did not involve pharmaceutical companies. Crucially as well as providing the drug that may be beneficial, pharmaceutical companies also provide the infrastructure and funding to conduct the studies.
As we know from the example of the UK Department of Health’s Dementias and Neurodegenerative Disease Research Network (DeNDRoN) having an infrastructure already in place greatly enhances our ability to conduct non-pharmaceutical led trials. The support from DeNDRoN includes ways of monitoring the trial while it is in progress and analysing the results when it has finished. The lithium study proved it is possible for neurologists and researchers to co-ordinate their own national clinical trials, the next step will be to work together to co-ordinate European clinical trials.
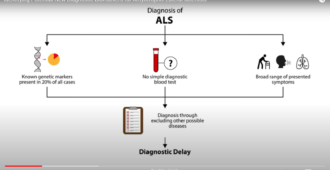
It was agreed that the first step to develop this infrastructure was ensure that we have more ways of sharing information on current research activities and a greater ability to react quickly to new opportunities. Linked to improved clinical trials is the availability of biomarkers that can be used and measured in a consistent way. Such biomarkers may improve the diagnostic process and therapeutic monitoring in MND. During the workshop a way of sharing the protocols for measuring biomarkers across Europe was agreed.
Delegates attended from the UK, Ireland, France, Germany, The Netherlands, Belgium, Italy, USA, Switzerland and Portugal representing a wealth of expertise: from running clinical trials; designing databases and registries; and developing a range of biomarkers from brain imaging to electrophysiology. As well as talking about improved collaboration, it was a great opportunity to hear about other research activities underway. It was also striking to note how well the proposed activities fit with the Association’s research strategy.
The workshop was organised by the European Neuro Muscular Centre (ENMC), who looked after us very well.




Belinda,
I’m interested in the workshop’s answer to “What would we do if another lithium came along?”.
Did the group discuss capitalizing online systems at all? Although done in a very different fashion, there has been recognition in the field that the patient-lead trial of lithium had some worthwhile directional findings as early as 2008 e.g. http://www.neurology.org/cgi/content/citation/75/7/586
Best wishes
Paul
Hi Paul,
Thanks for your comment. The question regarding the lithium trial was rhetorical rather than practical. It was a way for the participants of the workshop to consider what infrastructure neurologist led clinical trials (as opposed to pharmaceutical led clinical trials) would require. Thanks for the link to another way of conducting trials.
Belinda
What is the state of play with biomarkers? Have any been identified?
There are lots of biomarker studies underway around the world and the steps agreed at the workshop will help us move those into the clinic quicker. There are five posts on this blog that give you an update on ‘markers of disease progression’ which is one of the ways that biomarkers will be used in the future. You can find these posts by looking on the left hand column under categories. (These are underneath our latest twitter feeds).
I’m sure that we will receive an update on the latest news on biomarkers at the International Symposium on ALS/MND in December. Kelly will be posting on the blog from the conference, so look out for these mid-December!
Belinda