A recent paper published in the Journal Neurology: Neuroimmunology & Neuroinflammation has revealed new insights into how immune cells behave in MND. This work, which is the result of a collaboration between researchers across different universities, aimed to uncover more about the role of the immune system in MND progression.
The study used blood samples and information collected from people with MND as part of other research projects, such as AMBRoSIA, funded by the MND Association, and the ALS biomarkers study. This data was used to observe the behaviour of immune cells in MND and investigate if it could be related to disease progression. We chatted to Professor Andrea Malaspina and Dr Ozlem Yildiz about this work and what it means to people with MND.

What did this work focus on?
It is thought that changes occur in the immune system during the course of the disease and that immune cells may transform from being neuroprotective to being neurotoxic. Alterations in the behaviour of cells of the immune system have been suggested to contribute to disease progression and survival.
In this study, we analysed samples from people with slow and fast progressing MND separately. We looked at specific types of immune cells with a focus on cells that change with ageing to see how these changes might contribute to the disease. Symptoms of MND usually appear after the age of 50 and that is why ageing is thought to play a significant role in the disease. We also observe that the older the age of onset, the more rapid the disease progression and this may be due to the fact that as cells age, they may lose their function or start to function abnormally.
What are immune cells?
Immune cells act as a formidable barrier against harmful substances or organisms (like bacteria and viruses) that could cause disease. They also keep watch for any subtle changes in the behaviour of other cells and organs and respond to any damage. There are several different types of immune cells in our bodies which play various roles, but they all work together to protect us from lasting damage as a result of infection and injury. However, in some diseases the behaviour of the immune system can change, and cells can become senescent. When this happens, the immune system’s response may harm the very cells and functions that it was meant to protect in the first place.
This work focused on specific types of immune cells called lymphocytes, which are also known as white blood cells. These are cells that are produced in the bone marrow and move into the bloodstream to help target and respond to cells that are infected or damaged. There are two main categories of lymphocytes which are B lymphocytes and T lymphocytes.
What is senescence?
When immune cells age and no longer behave in a healthy way, they can adopt a state known as senescence. This is where the cell no longer replicates to make more cells but doesn’t die at this point when it usually would. Instead, it remains and releases toxic chemicals and antibodies that promote inflammation which can damage other cells around it. Immune cells normally develop senescence when they are exposed to repetitive harmful substances like during viral infections; they become exhausted and are unable to keep up with a healthy immune response.
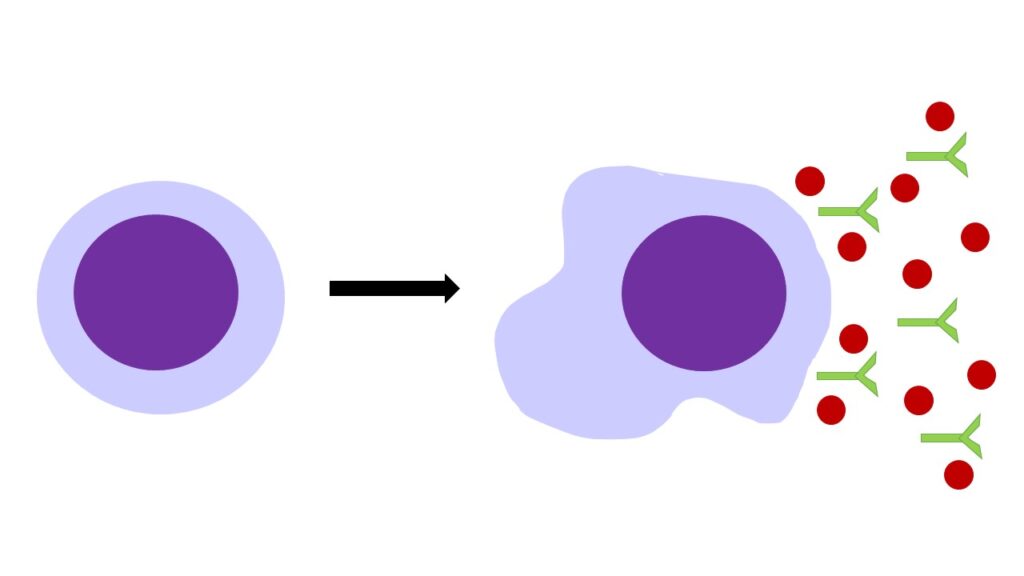
What did you find in this research?
We have shown that in people living with MND, there is a higher number of specific B and T lymphocytes showing features of senescence similar to what has been previously observed during the ageing process and in viral infections. We also found that higher amounts of these cells in the blood is linked to a worse prognosis of the disease. This suggests that changes in the immune system might be involved in the development and progression of MND.
There has also been another paper published by a group of American researchers which has shown similar findings in a type of genetic MND using animal models of the disease. This study underlines our findings and helps to provide further evidence that the immune system seems to play a role in motor neuron damage in MND.
What does this mean for people living with MND?
Our research has helped to highlight that there are several factors involved in motor neuron death in MND. It was previously thought that cell death was due to altered behaviour of certain proteins that become toxic in the disease, but this study has increased evidence that the immune system also contributes to motor neuron damage and death. It has been suggested that these faulty proteins may trigger a response from the immune system which is excessive and could harm surrounding neurons. A successful therapy may be about targeting these toxic proteins and the immune response they generate. Bringing immune cells out of this unhealthy, potentially damaging senescent state may be a part of this strategy.
What are the next steps for this work?
Although the study has helped to identify one of the immune changes that appears to be involved in MND, more studies are needed to uncover more about the immune cells that play a key role in MND and further understand the changes that occur. Further research in this area may help to increase our understanding of the mechanisms behind the disease which could lead to the identification of new biomarkers and potential therapeutic targets.
We wish to thank Andrea and Ozlem for taking the time to talk to us about this research.