The top-line results of the Modifying Immune Response and OutComes in ALS (MIROCALS) clinical trial were announced on 6 December 2022 at the 33rd International Symposium on ALS/MND. As part of the clinical trials session, the scientific leads Dr Gilbert Bensimon and Professor Nigel Leigh discussed the study design and outcomes (C03).
MIROCALS was an 18-month, randomised, double-blind, placebo-controlled clinical trial, investigating the use of low-dose Interleukin-2 (IL-2) in people living with MND. The MND Association was both a partner in the MIROCALS consortium, a 12-organisation strong partnership in Europe, and a funder of the trial alongside a number of charities that supplemented the core funding from the European Commission.
Inflammation in the brain and spinal cord is thought to influence the spread of damage throughout the brain and spinal cord and this has been linked to the speed of progression in MND. Our bodies can help to control inflammation through a type of immune system cell called a Regulatory T Cell (Treg). Research has shown that IL-2 can help to control Tregs and low doses have been shown to increase the number and function of Treg cells in the blood. A previous clinical trial, called IMODALS, investigated the use of low doses of IL-2 in people living with MND. The trial showed the number of circulating Tregs was increased in people living with MND.
RELATED TOPIC
Blog | 16 March 2022 | Eleanor Green
Symposium Spotlight: A closer look at IMODALS clinical trial outcomes
The MIROCALS trial recruited 220 people living with ALS (the most common form of MND) shortly after their diagnosis. The average person recruited into the trial had only been diagnosed just over one month previously. Upon starting the trial, participants also began treatment with Riluzole, the accepted standard drug treatment for ALS in the UK.
The trial looked at a range of endpoints, including survival, ALSFRS-R and whether the drug was interacting with the body’s biology as expected. They found that in those who received IL-2, the number of Treg cells in the blood increased. This is similar to the previous IMODALS trial, suggesting the drug is acting in the body as expected.
Although a 19% decrease in the risk of death at 21 months was found overall in those who received IL-2 which, this was not statistically significant. However, they did find there was a significant impact on survival when the effect of IL-2 was adjusted for an important biological factor that is known to predict survival.
What is statistical significance?
When a result is statistically significant, this means the result has a very low chance of occurring if there was no true effect in the study. When looking at this in terms of a drug trial, a statistically significant result means that the effect observed was due to the treatment, rather than due to chance. Statistical significance is often quoted using something called a p-value, which is an abbreviation of probability value. The lower the p-value normally means that the result is less likely to be due to chance alone. You can read more about p-values here.
ALS is a complex disorder with a number of causes and triggers. MIROCALS was designed to take this variability into account by integrating biomarkers into its design to help determine if the treatment was working. The key biomarker used was called neurofilament heavy chain (CSF pNFH) and it was measured by testing samples of cerebrospinal fluid. There are three different types of neurofilament: neurofilament light, medium and heavy. These neurofilaments can be thought of as Lego bricks, each of them a different size, and found in motor neurones as part of their scaffolding, keeping the structure secure. When the motor neurones become damaged, these neurofilament scaffolds can start to fall apart and you can then measure these in the blood or the cerebrospinal fluid.
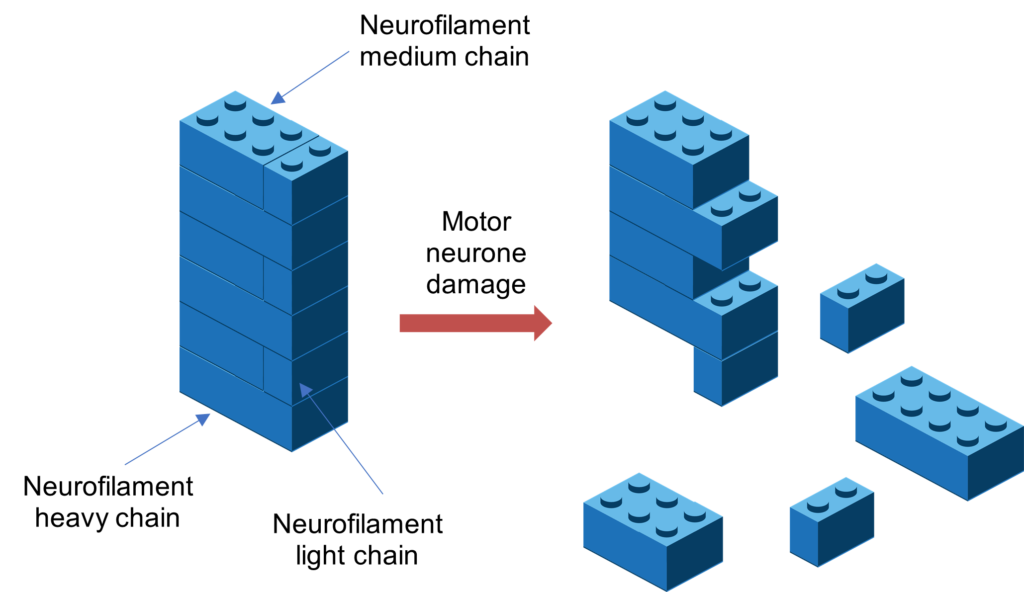
The trial was designed to include specific ‘pre-planned analysis’ (decided before the trial began), where participants were grouped based on their CSF pNFH levels. This pre-planned analysis was key to the personalised medicine approach of the trial, which was looking at whether some participants responded differently to IL-2. When they grouped those in the study population who had low to moderate levels of CSF pNFH levels (representing 80% of the population) which correlated with slower disease progression, they found that these participants had a significant decrease in risk of death of over 40%, at 21 months.
In addition to survival, the researchers also measured disease progression using the ALSFRS-R scale. Without adjustment for CSF pNFH, those who received IL-2 did have a small slowing in disease progression compared to those on placebo, but this did not reach statistical significance. However, in keeping with the survival data, the group of participants who had low to moderate levels of CSF pNFH did show a statistically significant slowing in disease progression.
These results provide encouraging evidence, from a randomised placebo-controlled drug trial, in support of immune system modification and neuroinflammation as viable targets for altering ALS disease progression. Further and deeper analysis of the results is now underway. At present IL-2 is not available for the treatment of ALS as it has not been approved for use, however the MIROCALS consortium are prioritising discussions with drug regulatory authorities on the next steps.
A key strength of MIROCALS has been the collaboration of leading European research groups in immunology, biomarker development and genomics. There remain many questions to address, but the wealth of data and samples accumulated are supporting ongoing research to better understand the factors that drive ALS disease progression. These will hopefully open the door to new therapeutic avenues and more personalised approaches to treatment, to deliver even more positive outcomes in future trials.
Professor Nigel Leigh (co-lead and chief trial investigator, of Brighton and Sussex Medical School)
We hope that the additional analysis will provide further evidence to support these initial encouraging findings and this research will help to accelerate the development of effective therapies for ALS and other motor neuron diseases. Trials such as these would not be possible without the continued support of the MND community, the dedication and commitment of researchers and the generosity and altruism of people living with MND, their families and carers.
The MND Association would like to thank the Garfield Weston Foundation, the J P Moulton Charitable Foundation, the Batchworth Trust, the David & Ruth Lewis Family Trust, the John Young Charitable Settlement and the Edwin George Robinson Charitable Trust for their generous support on this project.