As the research team are starting to get stuck into planning our next International Symposium on ALS/MND we are taking a look back at some of the incredible ALS/MND research presented at the 2021 event. As part of our Symposium Spotlight series, we delve into a poster presentation which took a closer look at the results of the IMODALS clinical trial, which investigated the use of Interleukin-2 as a treatment for ALS. These results are especially relevant whilst we are awaiting the readout from the larger follow up trial, called MIROCALS.
ALS is considered a multifactorial disease as several different factors are known to contribute to its onset and progression. One of these factors is neuroinflammation which is characterised by an increase in the immune response within the central nervous system. This increase in inflammation is thought to contribute to motor neuron damage in the disease. Regulatory T cells (Tregs) play a crucial role in regulating the body’s immune response by restoring the activity of the immune system to normal and helping to prevent unnecessary inflammation. However, there is growing evidence to suggest that the numbers of Tregs are dramatically decreased in those with ALS and they are less effective in suppressing the immune response, as shown by articles published in the Journal of Neuroimmunology and Acta Neurologica Scandinavica.
Interleukin-2 (IL-2) is a chemical messenger that is vital for the survival and function of Tregs. Several research studies have shown that when given at low doses, IL-2 can increase the numbers of these cells which leads to more efficient regulation of immune responses. For this reason, low-dose IL-2 has been proposed as treatment for ALS with the aim of expanding the number of protective Tregs and therefore reducing the excessive neuroinflammation seen in those with the disease.
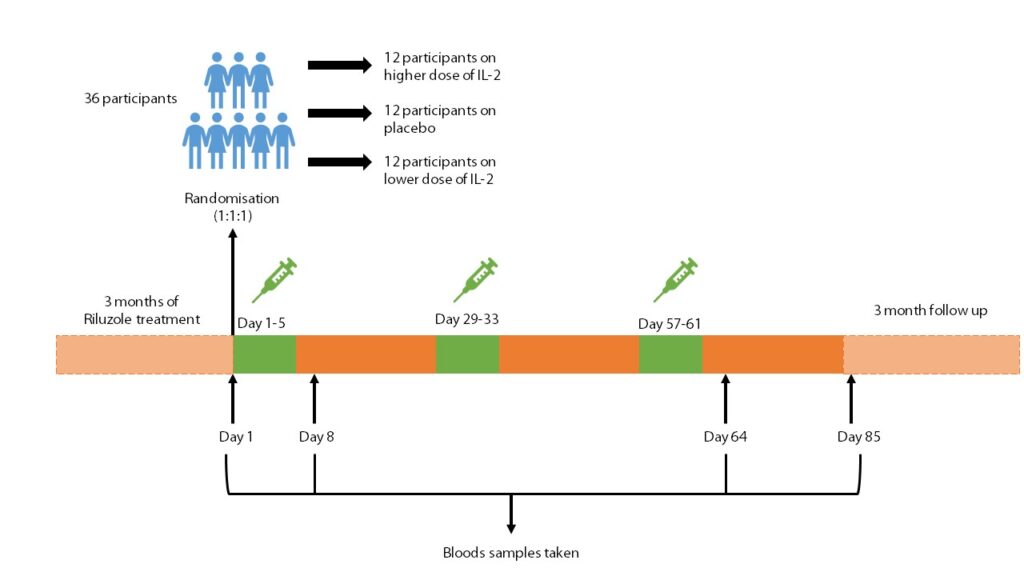
A pilot phase II clinical trial, Immuno-Modulation in Amyotrophic Lateral Sclerosis, named IMODALS (NCT02059759) was carried out to evaluate the safety and the effects of low dose IL-2. The trial recruited 36 ALS patients who were randomly assigned to one of three treatment arms (12 participants in each arm); two arms tested two different doses of IL-2 and one arm was a placebo (a substance which has no therapeutic effect). Participants received injections of either IL-2 or the placebo every day for 5 days. The treatment cycle was repeated every 28 days for 3 months (each participant received a total of three treatment cycles). Participants were then monitored for a further 3 months to see if effects of the treatment were lasting. The results of this study, published in Lancet EBiomedicine in 2020, showed that treatment with low dose IL-2 lead to an increase in the number and function of Tregs in those with ALS. You can read more about the results of the phase II trial in our previous blog on IMODALS.
Some recent work by researchers at the Sheffield Institute for Translational Neuroscience (SITraN), investigated changes that occurred in participants blood throughout the IMODALS trial. They studied blood that was collected from participants at multiple different time-points during the phase II trial, to observe which genes within immune cells were being switched on or off, and by how much, a type of analysis known as transcriptomics. By assessing the activity of genes, termed gene expression, the researchers were able to gather information on changes in immune system activity which could be due to low dose IL-2 treatment. This also allowed the researchers to establish whether there were any differences in the immune response between the two doses of IL-2 being tested and study long-term effects of the treatment on Treg number and function.
The results of this study, which was partly funded by the MND Association, were published in Brain Communications last year and were also presented at the 32nd International Symposium on ALS/MND (GEN-13) by Ilaria Giovannelli, a PhD student from the University of Sheffield. The presentation from the Symposium can be seen below.
The findings showed evidence of a dose-dependent trend which suggested that the number of Tregs increased more in those who were given the higher of the two doses of IL-2. As well as this, the gene changes that were found over the course of the 3-month trial indicated that the activity of the immune system was rapidly suppressed after the first cycle of treatment. The activity of genes responsible for the increasing the number and function of Tregs reached a peak after the third cycle of treatment with the highest dose of IL-2. However, the gene activity dropped once treatment stopped which suggests that continued treatment may be needed to help maintain the effects of IL-2 treatment on Tregs.
Interestingly, differences in Treg response to IL-2 were found between those with ALS, with a selection of participants showing a greater increase in the number of Tregs compared to others. These differences were associated with the activity of the immune system at the start of the trial, as those participants who were less responsive to IL-2 seemed to have higher levels of inflammation when beginning the trial. This suggests that excessive neuroinflammation may have a significant impact on the ability of the Tregs to react to low dose IL-2 and that measuring someone’s level of inflammation before treatment may help to predict their response to IL-2.
Finally, the researchers also proposed a predictive model based on measuring the levels of two different biomarkers, which they thought might be of use when predicting how someone with ALS may respond to IL-2 treatment. This model relies upon measuring the levels of two RNA molecules, called TLR9 and CD27 which relate to immune system function, before treatment begins. It was found that this model could be used to suggest the amount of Treg increase in participants following treatment with IL-2. This model could be particularly useful to identify those with ALS who would benefit the most from IL-2 treatment, providing the possibility of a more personalised approach to treatment in the future. However, further work is needed to validate this predictive model and ensure that it can accurately predict possible responses to IL-2 treatment.
The results of this follow-on study are promising and help to reinforce the previous findings from the IMODALS trial. They provide further evidence that IL-2 does increase Treg number and function, which may help to reduce the excessive inflammation seen in ALS. These findings support IL-2 as a potential treatment for some people with ALS, however further testing is needed to assess effects of IL-2 treatment on disease progression.
“Our results suggest that low-dose IL-2 is able to increase the protective Tregs and reduce the toxic neuroinflammation, a well-known ALS pathological mechanism“
Ilaria Giovannelli (PhD Student, SITraN, University of Sheffield)

Further analysis of IL-2 in a larger group of participants with ALS has been conducted in a phase 2 clinical trial called Modifying Immune Response and OutComes in ALS (MIROCALS). This larger trial (NCT03039673), partly funded by the MND Association , aimed to investigate the impact of IL-2 treatment on disease progression and survival in over 300 people with ALS. It is also hoped that the MIROCALS trial will help to validate the predictive model developed by researchers at SITraN.
“The predictive model is of crucial importance as, if validated in the larger MIROCALS trial, a precision medicine approach could be used to specifically treat patients who would benefit the most from this drug. ”
Ilaria Giovannelli (PhD Student, SITraN, University of Sheffield)
The MIROCALS trial has now ended, and the data is currently being analysed. Results from this study are expected in 2022. To find out more about the MIROCALS trial please visit our website or our previous MIROCALS blog.
To find out more about the research presented at the 32nd International Symposium on ALS/MND, please see our Symposium Blogathon or published online abstracts.
We would like to thank Ilaria Giovannelli for her contribution to this blog.