QurAlis, a biotechnology company focused on developing precision medicines for MND and other neurodegenerative diseases, has announced the opening of a clinical trial in the UK. This trial of a gene therapy, called QRL-201, is a phase 1 trial in people with MND and will test the therapy to determine whether it is safe and well tolerated by participants. QRL-201 is designed to increase levels of a protein called Stathmin-2 in people with MND.
What is Stathmin-2?
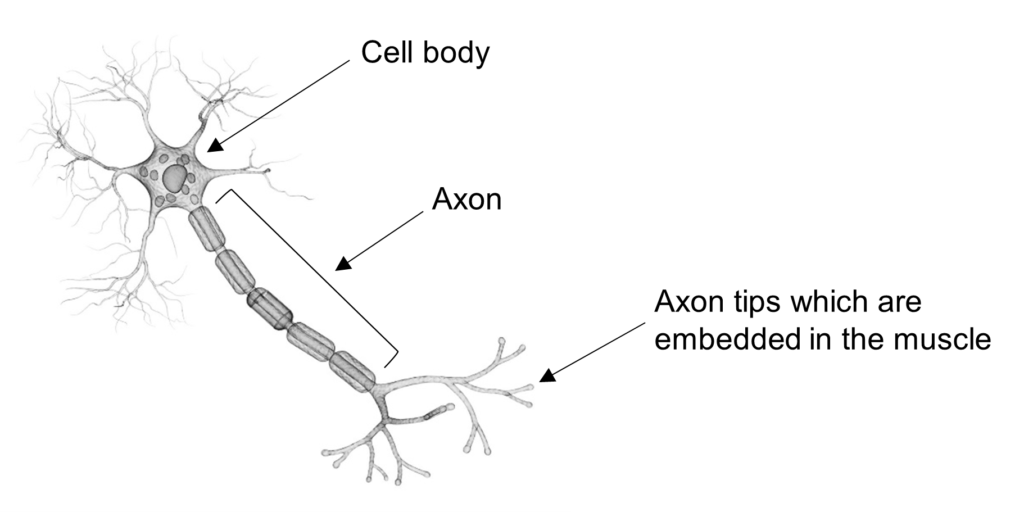
The Stathmin-2 protein is crucial to the process of repairing damage to neurons and is also heavily involved in maintaining the correct structure of axons. Axons are long, thin extensions of motor neurons that arise from the cell body and project out to connect to other brain and muscle cells. The motor neuron uses the axon to send electrical signals to the muscle to trigger muscle contractions.
RELATED POST
Blog | 23 August 2022 | Research Development team
Travel disruptions: transport along neurones in MND
How is Stathmin-2 involved in MND?
The Stathmin-2 protein is usually found in high levels in motor neurons due to its role in making sure the axons remain functional so that messages can be sent along them. A paper published in 2019 helped to highlight the important role that Stathmin-2 may play in the development of MND. Researchers from Harvard University found evidence that levels of Stathmin-2 are regulated by another protein, TDP-43, which has long been associated with MND. In MND, TDP-43 moves outside of the nucleus of the cell and into the cytoplasm where it forms toxic clumps. The loss of TDP-43 from the nucleus means that TDP-43 can’t work as it normally would. This loss of function was shown to lead to a decrease in levels of Stathmin-2 in MND.
RNA is a photocopy of our DNA and some of it is used as instructions to make proteins. The parts of RNA that are needed to make the protein are known as exons and the parts that don’t contain instructions are called introns.
TDP-43 in the nucleus usually binds to part of the RNA used to make the Stathmin-2 protein. This interaction of TDP-43 helps to regulate the removal of introns from the protein instructions, leaving just the exons, that are needed to make the protein.
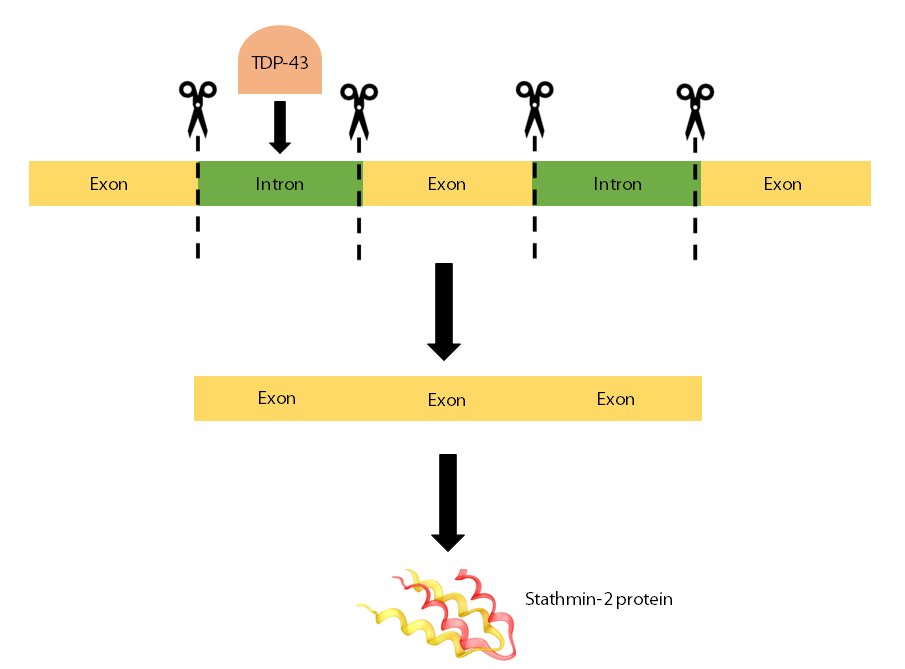
However, when TDP-43 is no longer in the nucleus, and doesn’t function as it should, an intron gets incorrectly included in the instructions. This means that the instructions to make the protein are a lot shorter than they should be and are destroyed by the cell so the Stathmin-2 protein isn’t made. This intron contains a stop signal which tells the cell to stop making the RNA instructions needed to make more Stathmin-2 protein. Both of these effects work together to cause less functional Stathmin-2 to be made.
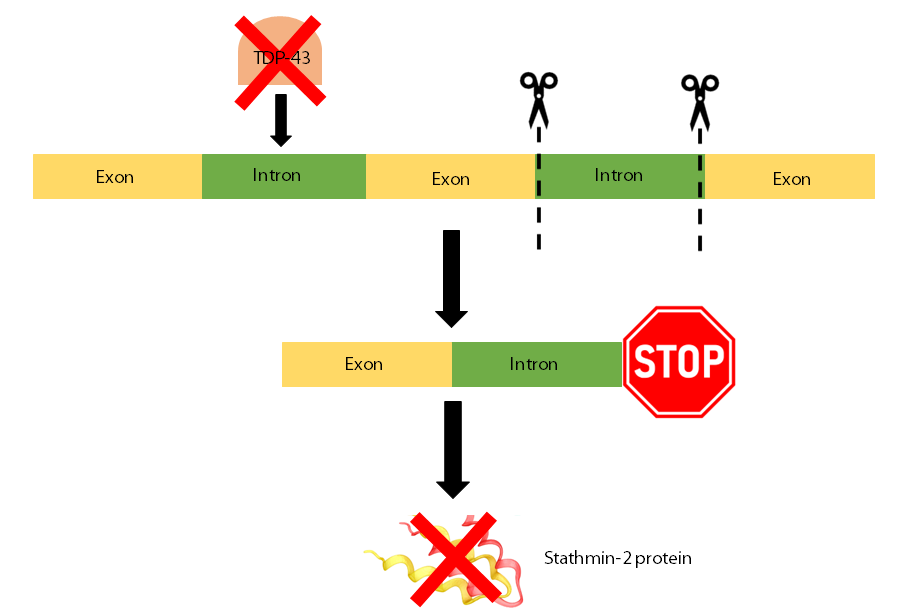
These lower levels of Stathmin-2 are thought to reduce the repair of damage to neurons and cause the axons to degrade. This means that signals can no longer be sent to the muscles or other neighbouring cells. This loss of communication between the neurons and muscles may contribute to the muscle weakness and wasting that is seen in MND.
How is QRL-201 thought to work?
QRL-201 is an antisense oligonucleotide (ASO) which is designed to increase levels of the Stathmin-2 protein by preventing the intron from being included in the RNA instructions. Stopping the intron from being incorrectly included in the protein instructions may help to restore levels of functional Stathmin-2 protein.
Previous research in human cell models of MND has demonstrated that restoring levels of Stathmin-2 could help correct the repair response in neurons and rescue the damage to axons, even in the presence of faulty TDP-43.The promising findings from this pre-clinical research have helped to progress QRL-201 to a phase 1 clinical trial to test the potential treatment in people with MND.
A new phase 1 trial
QRL-201 is being tested in a Phase 1 trial called ANQUR which is aiming to assess it’s safety and tolerability in people with MND. Phase 1 trials usually test a potential treatment in healthy volunteers, so it is exciting that this possible therapy is being tested in people with MND and gives the opportunity to test it earlier than usual in a clinical trial.
The trial will evaluate several different doses of the potential treatment against a placebo (dummy drug) in up to 64 people with MND across multiple countries, including the UK, Canada and several countries in Europe. QRL-201 will be given through injections into the fluid surrounding the spinal cord, with five treatments given over a period of three months.
Participants will be split into small groups, called cohorts, testing different doses of QRL-201. Six participants in each cohort will get a dose of QRL-201 and two participants will be given the placebo. Participants will be randomly assigned to receive the experimental treatment or a placebo and neither the researcher nor the participant will know which they are given. The ANQUR trial is recruiting more people in the treatment group of the trial compared to the placebo (in a 6:2 ratio in each dose group).
The trial will help to assess whether QRL-201 is safe for people with MND, with the primary outcome of the trial being observations of any side effects or worsening of symptoms associated with the treatment. Secondary endpoints of the trial include several measurements of amount of the drug in the blood, which helps to provide information on exactly how it moves into, through and out of the body.
How can I get involved in the trial?
ANQUR is a multi-centre clinical trial which will recruit participants in the UK, Canada and Europe. It will recruit in the UK at Sheffield and King’s College London MND care and research centres.
Please note that opportunities to take part in this clinical trial are limited to each cohort and are currently by invitation only. Each cohort is recruiting one by one. This means that once one cohort has fully recruited, there may be a waiting period before the next cohort opens for recruitment.
If you’re interested in taking part in this trial or any other trial at these sites, please contact the study teams at:
The pre-clinical data for QRL-201 look promising, but it is important to wait for the results of this Phase 1 clinical trial to see if it is safe for people with MND. We will continue to follow the ANQUR clinical trial and will keep the MND community informed of its progress. You can find out more about QRL-201 and ANQUR on our website here.
* The information in this blog was correct at the time of publication