Hi, I’m Ben, a researcher working at Sheffield University. I attended last year’s International Symposium on ALS/MND as one of the Symposium Communication Ambassadors and this is one of the most interesting pieces of work I saw.
MND is an incredibly complex disease, with many factors contributing to the death of motor neurons. The way we study this complex disease varies. A lot of studies are performed in a clinical setting, meaning on people with MND; many are performed in vivo, meaning in living organisms (e.g., mice); however, the vast majority of investigations are performed in vitro which means they take place outside of a living organism, which could mean in a test tube, Petri dish or other lab vessel.
When researching human disease virtually all experiments will at some stage involve cells that are either taken directly from people with the disease in question, or cells that have been manipulated to recreate that disease. These types of experiments are quicker, cheaper, and simpler methods (compared to clinical or in vivo work) to investigate how our cells might change in response to disease. In vitro work provides a basis for future work that is performed in vivo and eventually the clinic as it allows us to justify the more expensive, more involved, and more time-consuming work.
The main disadvantage to this method is that findings from in vitro work don’t always translate perfectly from the lab bench to the clinic and that translation is key to bringing solutions to people with MND as soon as possible. There are two major reasons for this, firstly that cells don’t grow alone in the body, they are one part of complex systems of cells, with multiple different cell types growing in close proximity to each other, carrying out various functions and interacting with each other constantly. The other major factor is that in the lab, cells are grown on flat surfaces (often petri dishes), effectively a 2D structure, but in the body, cells grow in 3D space, so 3D in vitro investigations are more representative of how cells would grow in the body.
Researchers are therefore constantly working to recreate these conditions more accurately. One way of doing this involves growing multiple different cell types together in materials that allow them to grow in 3D spaces. This was the aim of Dr Marianne King at the Sheffield Institute for Translational Neuroscience, part of the University of Sheffield, working in collaboration with researchers at the A.I. Virtanen Institute for Molecular Sciences at the University of Eastern Finland, the University of Applied Sciences and Arts Northwestern Switzerland, Uppsala University, İstanbul Medipol University and King’s College London. The project was funded by the Joint Programme – Neurodegenerative Disease research initiative all part of a consortium led by Professor Mimoun Azzouz. Her poster at the 34th International Symposium on ALS/MND in Basel was one of the winners of the biomedical research poster prizes, who’s poster was in competition with the several hundred other poster presenters attending the event.
For MND, in vitro work often focuses on individual cell types. A lot of work is performed on neurons, as would be expected, however, there are many different cell types affected in those with MND, including astrocytes and microglia which are important neuronal support cells.
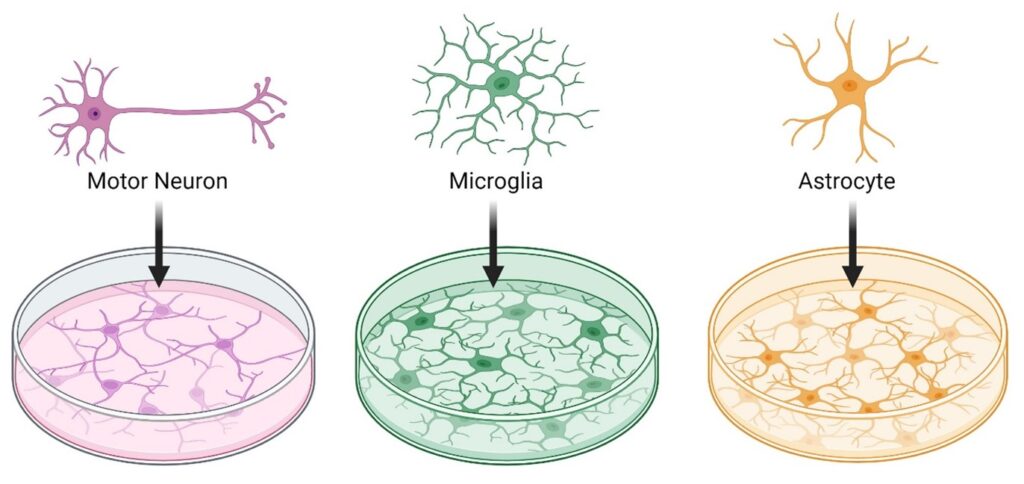
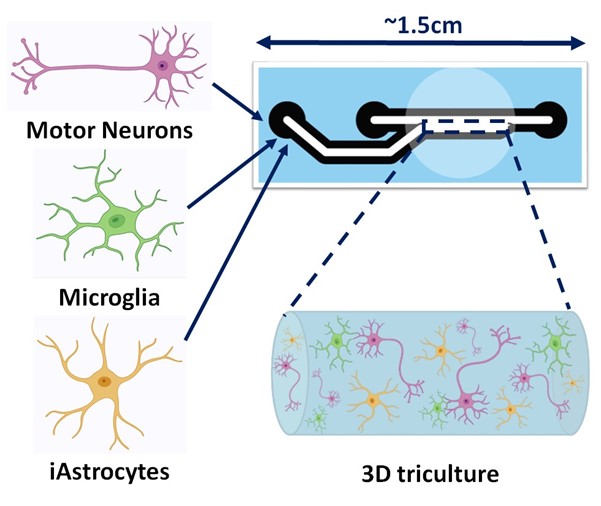
Marianne’s work involved taking astrocytes and microglia and growing them with neurons in a substance that allows cells to grow together in a 3D space, mimicking the environment cells would normally grow in within the body. The process may sound simple but is actually lengthy and challenging, with rigorous testing at every stage to ensure that cells grown in this way are representative of the human body.
First of all, you can’t just take brain cells (the neurons, astrocytes and microglia) in question directly from patients. Marianne and her collaborators therefore first took skin cells from people with MND and then reset them, to transform them into a type of cell know as a stem cell that can be manipulated to form any type of cell that is required. Once the stem cells were generated, they had to be manipulated to form the target cells, in this case neurons, astrocytes, and microglia. After generating these cells, Marianne had to then confirm that the cells were able to survive in 3D cultures. She therefore initially took only neurons and astrocytes and then neurons and microglia and tested whether they were able to survive in the 3D cultures together. As we can’t picture cells with the naked eye, Marianne used high-powered microscopes to view her cells in these cultures and confirm that they were able to survive.
It is also important to understand whether the cells in these cultures behave as we would expect them to in the human body. The role of microglia is to act as a biological vacuum cleaner, hoovering up foreign material that could be harmful to other cells around it. Marianne showed that the microglia were still able to carry out this function by making them hoover up light emitting particles that we are able to see inside the microglia. Again, this is important as it shows the cells are behaving as they would in their usual environment – the human body.
After all these steps were carried out, the final stage was to actually culture all three cell types together in the 3D culture. Marianne did this and then confirmed that the cells were again able to survive all together by dusting off her trusty high-powered microscope. This means that she now has a model which far more closely resembles what we would see in the human body compared to other in vitro models.
This important work will now allow Marianne, and other researchers investigating MND, to perform investigations in vitro that are more representative of what we would be likely to see in a person with MND. Being able to use this model to improve in vitro studies may enhance the translation of work we do in the lab into the clinic and effectively streamline how we develop potential treatments for MND in the future.
We would like to thank Ben for taking the time to write this blog and also for being a Symposium Communications Ambassador. You can follow Ben on Twitter/X here.