On 25th April 2023 Tofersen (now known as Qalsody in the US), a treatment for SOD1 MND was approved by the Food and Drug Administration (FDA) in the United States. The FDA approval in the US is a welcome step forward in the fight against MND and further highlights the commitment and dedication of the research community in finding effective treatments. Approvals such as these would not be possible without the generosity and selfless commitment of people living with MND, their families and carers. Most countries around the world have their own regulatory agencies. We now await the decision from the European Medicines Agency to see if the treatment could be approved in the UK and Europe
In this blog we take you through the journey to the FDA decision for Tofersen, answering some frequently asked questions, as well as explaining what this approval means to people living with MND in the UK.
What is Tofersen?
Tofersen is an antisense oligonucleotide (ASO) that targets a specific genetic mutation, known as SOD1, known to cause MND. SOD1 mutations account for around 2% of all MND cases. People living with MND who have a mistake in the SOD1 produce faulty SOD1 protein, which is toxic to motor neurones. Tofersen targets the genetic instructions for the faulty SOD1 protein, stopping it from being produced. You can read more about how Tofersen works in a previous blog.
Has the drug been tested in a clinical trial?
Tofersen has been tested in several clinical trials. A Phase 1/2 trial started in 2016 and demonstrated proof-of-concept of Tofersen. This led to a larger Phase 3 trial, known as VALOR, to take place.
How was the VALOR Phase 3 trial designed?
The trial was designed as a randomised, placebo controlled, double blind trial for 28-weeks. This meant that the participants were either randomised into the treatment group or the placebo group (dummy drug) for 28-weeks and neither the investigators or the participants knew who was in which group (double blind). The primary outcome of the trial was MND progression as measured by the ALS functional rating scale (ALSFRS-R) over the 28-weeks.
An open-label extension was also designed into the trial. After 28-weeks all the participants were given the option to have the treatment, regardless of whether they were originally in the placebo or treatment group.
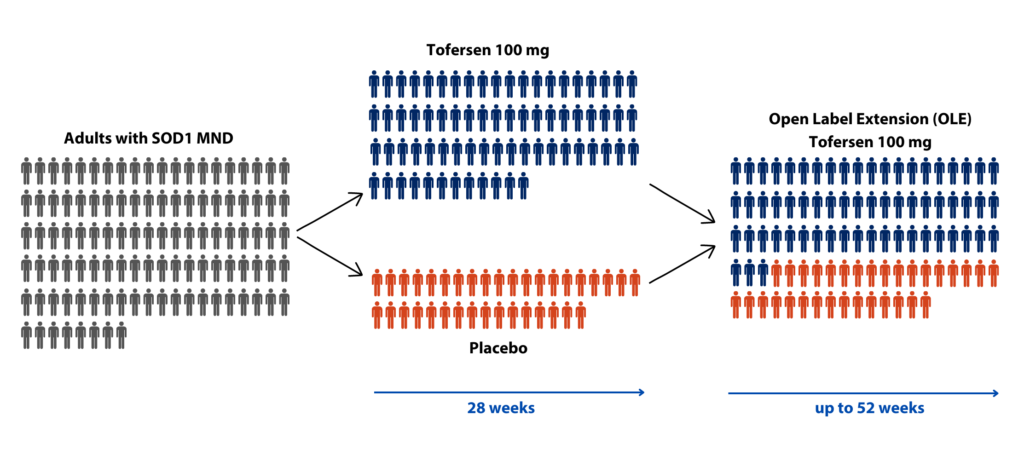
Who took part in the VALOR Phase 3 trial?
Participants were enrolled in the trial if they had a mutation in the SOD1 gene. They were randomly assigned in a 2:1 ratio of Tofersen to placebo. A total of 108 participants were randomly assigned to the treatment group (72 participants) or placebo (36 participants). Participants who completed the 28-week study were eligible for the open label extension. Of the original 108 participants who were randomised, 95 entered the OLE. This setup resulted in two groups: an ‘early start Tofersen’ group who received the drug throughout all of the study (for 52 weeks), and the ‘delayed start Tofersen’ group that first received placebo (for 28 weeks) but then went on to receive the drug (from 28 to 52 weeks).
What were the results of the VALOR Phase 3 trial?
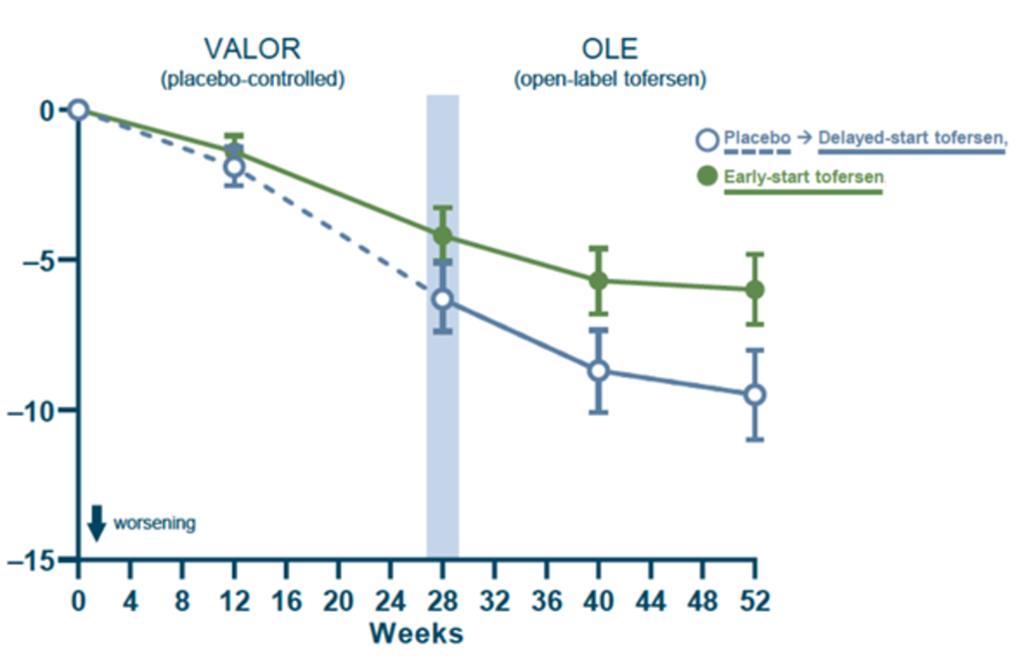
The primary outcome of the trial was the change in the ALSFRS-R total score from baseline to week 28. Essentially, the researchers were looking to see if the rate of disease progression changed over the 28 weeks. MND symptoms progressed in both groups indicated by the fall in ALSFRS-R in both groups. Unfortunately, after 28 weeks analysis of the results showed that there was no statistically significant difference in the rate of MND progression (measured by ALSFRS-R) between the treatment group and the placebo group.
Participants continued to be closely monitored as they moved onto the open label extension phase of the trial. Over the 52-weeks MND symptoms continued, indicated by the fall in ALSFRS-R in both groups. However, after 52-weeks there was a difference of 3.5 points between the ‘early start Tofersen group’ (green line) and the ‘delayed start Tofersen group’ (blue line). This difference in the ALSFRS-R between the groups represents a slowing in the rate of disease progression for those in the group who had taken the treatment since the start of the trial. This was also shown in other secondary measures of disease progression, such as measures of respiratory function and muscle strength.
What effects did Tofersen have on markers of disease activity?
During the trial, the researchers took samples of blood and spinal fluid from the participants to measure two biological markers which could indicate the effectiveness of the treatment. These were:
- Levels of SOD1 protein – this protein is toxic in people living with SOD1 MND
- Neurofilament light chain – a potential marker of the disease.
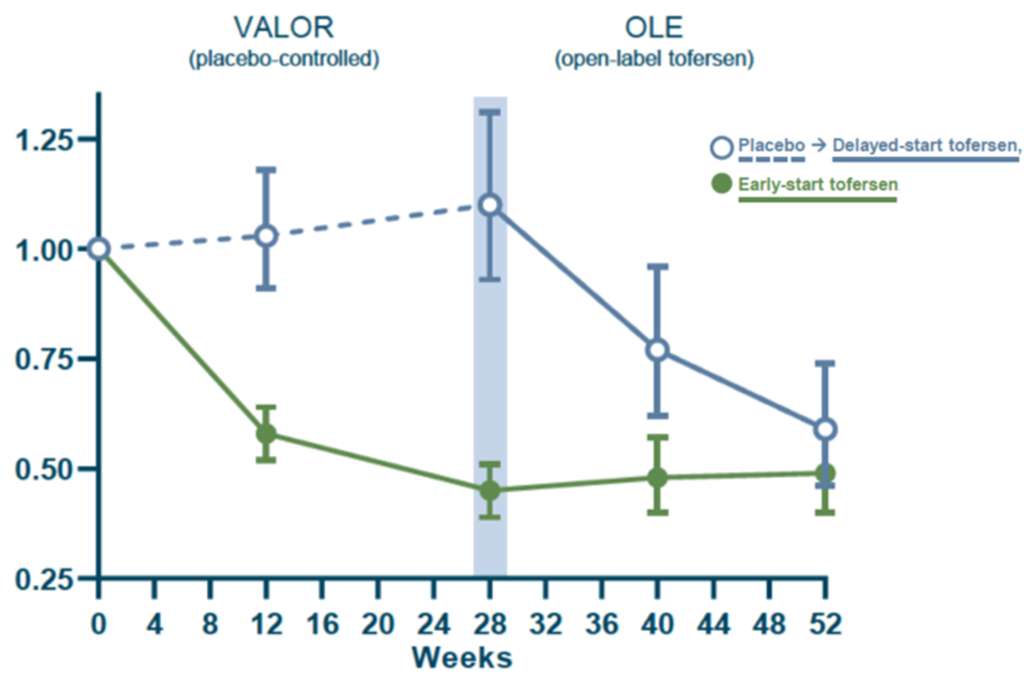
For both the initial 28-week trial and the open label extension, treatment with Tofersen was found to reduce SOD1 protein levels. This suggests that Tofersen is engaging with the biology it was designed to and could be working as expected. Participants who were initially randomised to the placebo did not see a drop in their SOD1 protein levels until they were given the treatment in the open label extension.
It was a similar story when looking at the levels of neurofilament light chain (NfL). NfL is a potential marker of nerve damage and appears in the blood as nerve cells are broken down. A reduction in the levels of NfL indicates a possible reduction in nerve damage and disease progression. For both the initial 28-week trial and the open label extension, treatment with Tofersen (green line) led to a reduction in NfL. However, participants who were initially randomised to the placebo (blue line) did not see this reduction in NfL until they were given the treatment in the open label extension. The FDA highlighted that the approval of Tofersen was based on the reduction in neurofilament light chain observed in the trial.
What impact did Tofersen have on survival?
In general participants who were in the ‘early start Tofersen group’ lived longer than those who were in the ‘delayed start Tofersen group’. Therefore, a person’s chance (probability) of survival was greater if they received the treatment earlier and for longer.

RELATED POST
Blog | 10 June 2022 | Charlotte Roy
VALOR Biogen’s Tofersen trial: a look at the open label extension data
How can I access the drug?
Biogen, the pharmaceutical company who developed Tofersen, have set up an Early Access Programme to allow people living with SOD1 MND to get access to Tofersen without being on a clinical trial and before it has been approved. This process can be complicated, and a neurologist needs to go through the approval process with Biogen. The process for neurologists is available on Biogen’s website. If you are interested, please contact your neurologist.
Can I take part in a clinical trial testing Tofersen?
There is a clinical trial investigating Tofersen currently running in the UK. However, this trial is recruiting people who have particular SOD1 gene mutations but are not currently showing symptoms of MND and are considered pre-symptomatic.
The trial data from VALOR suggests, as might be expected, that the earlier a potential therapy can be given the more beneficial a treatment may be. This Phase 3 trial is looking to determine the optimal timing to begin treatment with Tofersen. It aims to investigate whether pre-symptomatic treatment can delay the onset of symptoms and slow disease progression when symptoms do develop.
ATLAS is recruiting in Sheffield in the UK. For more information on how to get involved, head to our website or contact:
- Lee Tuddenham, Lead Research Nurse, on lee.tuddenham@nhs.net.
RELATED TOPIC
Website | Clinical Trials
Find out about treatment trials recruiting in the UK
How can I find out if I have a SOD1 gene mutation?
Tofersen has been designed specifically for people living with MND who have a mistake in the SOD1 gene. A genetic test can be performed to find out if a person has this mistake.
If you are interested in having a genetic test, speak to your healthcare provider. It is important to acknowledge that having a genetic test is a personal decision and the potential implications for the individual and wider family should be carefully considered, along with appropriate genetic counselling.
What does approval look like in the UK?
The European Medicines Agency (EMA), which determine approvals for the EU, including Northern Ireland, are currently reviewing the data around Tofersen.
The regulator for Great Britain (England, Wales and Scotland) is called the Medicines and Healthcare products Regulatory Agency (MHRA). Since Brexit, a process (known as the European Commission Decision Reliance Procedure) has been put into place so that the MHRA may rely on a decision taken by the EMA on the approval of new treatments. Biogen are expecting that Tofersen will go through this regulatory pathway in Great Britain.
Regulatory approval of a new treatment in the UK does not automatically mean it will be available on the NHS. To determine if the treatment will be available on the NHS, it must be assessed by the National Institute for Health and Care Excellence (NICE) and the Scottish Medicines Consortium (SMC). NICE and SMC advise the NHS on whether a new treatment is cost effective. During their evaluation they review evidence from clinical trials, assess the benefits and quality of life the treatment would provide and weigh these against the cost of the treatment. They also get input from experts and those affected by the disease to help them make this decision. Tofersen is currently in the early stages of this process with NICE, which is not expected to finish until mid-2024 at the earliest.
Tofersen will only then become available on the NHS once it has received positive decisions from both the regulators and NICE/SMC.
We are in contact with Biogen and will keep the community informed as we hear more.
Moving forward
With over 90 MND clinical trials currently in preparation or in progress globally, the news of the FDA approval provides hope for the pathway to approval for other treatments under investigation in trials. With the current momentum in MND research showing no signs of stopping, we are hopeful that more treatments will be found to add to the treatment options available. None of this progress is possible without the continued support of the MND community, the dedication and commitment of researchers and the generosity and selflessness of people living with MND, their families and carers.
If you still have questions regarding Tofersen and the ATLAS trial, please get in touch with the research team on research@mndassociation.org.