Introduction
A genetic change in the C9orf72 gene is the most common genetic cause of motor neurone disease (MND), accounting for 40%-50% of people with familial MND and 5%-10% of people with sporadic MND. This genetic change in C9orf72 can also lead to behavioural symptoms of frontotemporal dementia (FTD), in some people with MND (around 15%). Researchers from the Paris Brain Institute have been looking into the C9orf72 related MND and its relation to FTD in this study.
What did this work focus on?
Mutations in the C9orf72 gene can lead to improper production of C9orf72 protein, resulting in a lower than normal amount being produced.
The exact way in how a decrease in C9orf72 protein causes these symptoms is relatively unknown, therefore researchers are interested in investigating this particular mutation. Recent studies have shown that a decrease in the C9orf72 protein plays a role in the development of MND symptoms in human neurones and animal models.
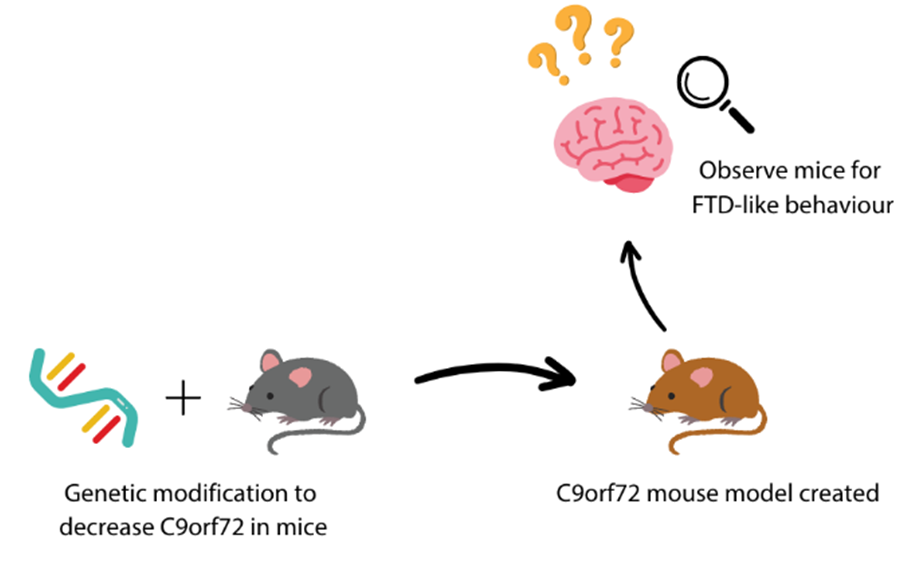
Through the use of cutting edge technology to modify the genes of mice, the researchers in this study decreased the amount of C9orf72 protein in mouse models to mimic the mutation and symptoms observed in those with C9orf72 related MND. This mouse model was created with the intention to provide MND researchers with a more accurate animal model of the C9orf72 genetic change seen in humans for further MND research.
What did the study uncover?
Upon observing post-mortem brain tissue at 23 months of age, it was found that C9orf72 mice showed no loss of neurones nor neuronal damage when compared to mice with no C9orf72 reduction (control). This is unlike people living with MND, where neuronal damage is seen in the brain. The spinal cords of the C9orf72 mutated mice showed no decrease in the number of motor neurones when comparing with the controls. Interestingly, though no neuronal damage was observed, the mice performed worse in a grip test to determine muscle strength and the connections between their neurones and muscles had a different appearance when compared to their control counterparts. This may suggest that although the neurones are intact, the connections between nerve and muscle were not formed correctly, leading to a decrease in strength.
The clumping of proteins p62 and TDP-43 are both hallmarks of C9orf72 related MND. Typically, p62 and TDP-43 reside in the nucleus of the cell, the area of the cell that contains DNA. However, in those with C9orf72 related MND, the proteins clump together outside of the nucleus which are toxic to neurones. Though no neuronal damage was seen in the mice, the characteristic clumping of p62 and TDP-43 was observed outside the nucleus. The study suggests that this may be due to a minimal amount of proteins sticking together, meaning there wasn’t a large enough amount nor enough time had passed to cause neuronal death.
After the researchers conducted behavioural tests on the mice, it was found they showed symptoms of FTD as early as 5 months of age. When a swim test was undertaken, the mice stayed still for longer periods of time compared to their control counterparts, which is a display of apathetic behaviour. They were also unable to tell the difference between a new mouse and a mouse they met before in a sociability test, showing they had troubles with recognising familiar mice. Both apathy and troubles with recognising faces are behavioural symptoms seen in people with MND- FTD, showing the mouse model does indeed mimic the human symptoms.

RELATED POST
Blog | 20 April 2023 | Eleanor Green
Measuring cognitive and behavioural changes in MND
What does this mean for people living with MND?
This study has managed to generate a mouse model that recreates the symptoms of FTD in C9orf72 related MND. The exact way changes in C9orf72 leads to MND is yet to be fully understood, which is why an animal model that displays symptoms as close as possible to those seen in people living with MND is crucial for MND research. Further studies using this mouse can be used to understand the biological pathways that the C9orf72 mutation disrupts. Potential biological pathways to target with drugs could possibly be discovered in future studies, leading to the development of potential therapeutics for MND.
What are the next steps in this work?
Further work will need to be conducted to fully understand how mutated C9orf72 gene causes MND. Though a decrease in C9orf72 protein lead to FTD-like symptoms and the hallmark build up of p62 and TDP-43 outside the nucleus, there was no evident neuronal damage found in the dissected brains and spinal cords of the mice. This may mean that other factors are needed to trigger full blown MND symptoms which requires further investigation.