Recent research, led by Professor Guillaume Hautbergue at the University of Sheffield, has found a potential new method of preventing nerve cell death in the most common genetic form of MND. This new method could, with further studies, lead to the development of a potential therapy for C9orf72 MND, and a type of dementia called frontotemporal dementia (FTD). The researchers also hinted that one day this potential therapy might be administered through non-invasive methods, such as a nasal spray.
What is the most common genetic form of MND?
Around 5-10% of people living with MND have a mistake in the C9orf72 gene. The mistake in the gene is known as a ‘repeat expansion’, which means a very small part of the genetic sequence (instructions for proteins) is repeated over and over again. The repeat sequence in the C9orf72 gene causes the instructions to be wrong and leads to the formation of toxic proteins. A good way to think of this is like the instructions for building a car, where one word in the instructions is repeated thousands of times. This causes confusion on the production line and ultimately problems in the operation of the car. In MND, the toxic proteins made as a result of the ‘repeat expansion’ in the C9orf72 gene are known as dipeptide repeats or DPRs and are harmful to neurones.
How are toxic (dipeptide repeat) proteins made?
Our genes are the instructions for making proteins, which are needed for a wide variety of different functions in our bodies. These instructions are held in the DNA within the command centre of the cell which is called the nucleus. In order for a specific protein to be made, a copy of its instructions has to be created and then transported out of the nucleus into the cell’s cytoplasm, which is where the cells protein factory is. The copy, known as RNA, can then be used as a precise instruction manual to make the correctly functioning protein.
Usually, cells have several mechanisms in place to prevent potentially harmful genetic mistakes being made into harmful proteins. However in the case of C9ORF72 MND, the harmful repeat sequence in the C9orf72 gene is carried over in the RNA copy. This copy is then transported into the cytoplasm of the cell and the harmful toxic proteins are made.

RELATED TOPIC
Blog | 5 July 2017
Closing the door on toxic proteins – new clues in understanding a genetic form of MND
How are the faulty instructions transported?
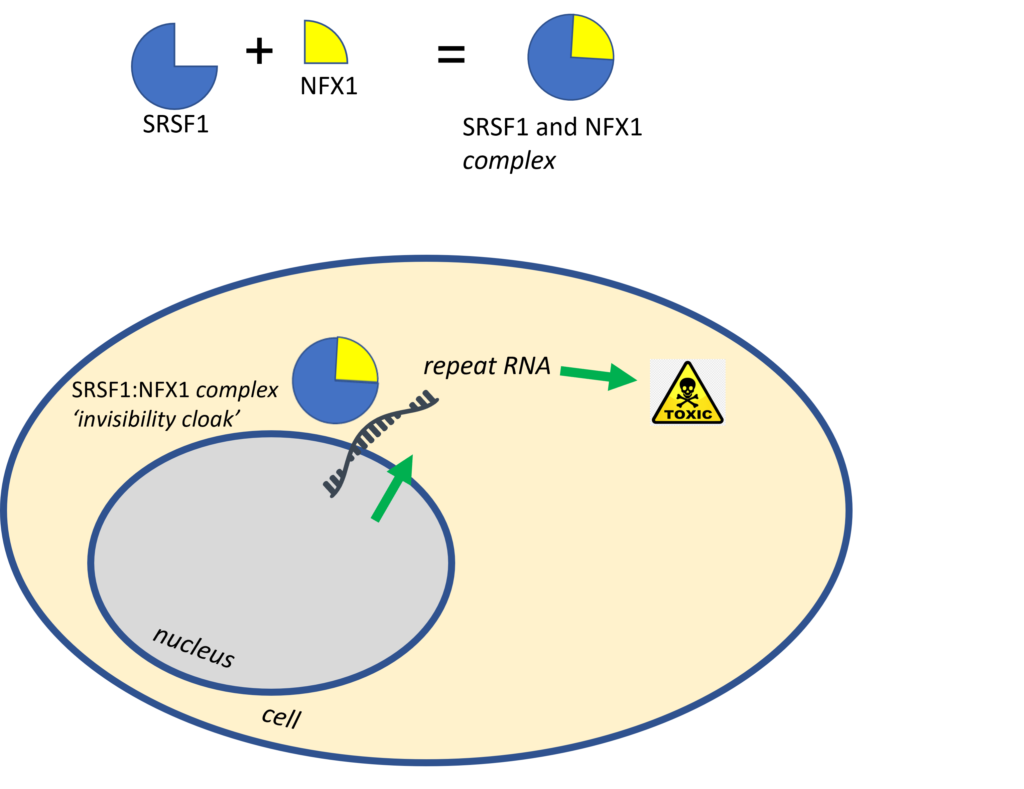
A protein called SRSF1 is involved in transporting the faulty instructions out of the nucleus and is therefore critical to the processes that make the toxic proteins. Previous work, supported by the MND Association, found that reducing the amount of SRSF1 protein led to a reduction in the amount of toxic proteins being made. The team looked further into how SRSF1 works and found it wasn’t acting alone – it has a partner in crime known as NFX1. SRSF1 and NFX1 join together allowing the faulty instructions to pass through the pores in the nucleus and then go on to make the toxic proteins.
We could think of this complex as forming an ‘invisibility cloak’. When SRSF1 and NFX1 join together they allow repeat RNA to sneak out of the nucleus to make toxic proteins instead of being detected and destroyed.
Can the transport of the faulty instructions be stopped?
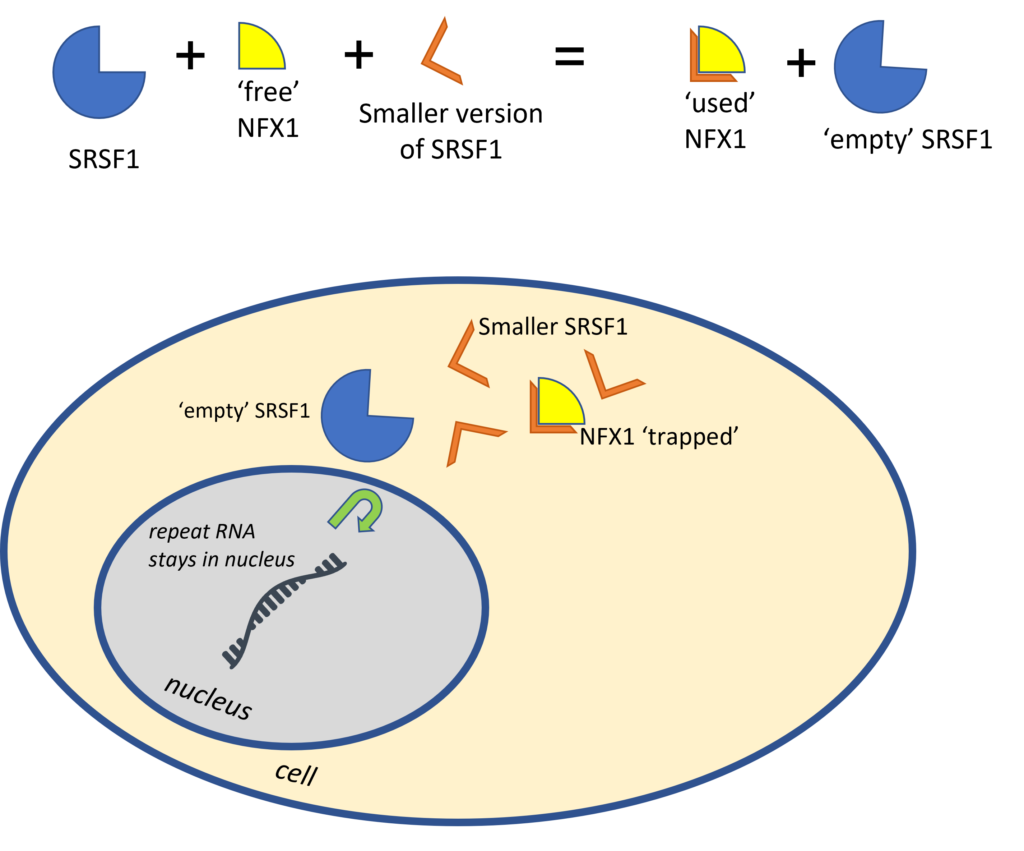
In this new research, the researchers found a way to make it less likely for SRSF1 and NFX1 to be able to join together. Without the SRSF1:NFX1 complex being able to form, there would be no transport of the faulty instructions out of the nucleus and no toxic protein would be made. To do this the researchers made a small specific part of SRSF1, which contains just the part that binds to NFX1 (known as the SRSF1 binding site). To NFX1 this smaller version of SRSF1 looks and acts very similarly to genuine SRSF1 and therefore NFX1 sticks to it. The researchers add enough of the smaller version of SRSF1 to bind to and effectively ‘soak up’ all the available NFX1, so that no natural SRSF1 and NFX1 joins together. Without the ‘invisibility cloak’ the faulty instructions are not transported out of the nucleus. Therefore, resulting in a reduction in the number of toxic proteins being made.
The introduction of the smaller version of SRSF1 into the cell, outside of a laboratory experiment, can be tricky. The potential therapy needs to be delivered to the right cells within the body. In the case of MND, this would be the central nervous system (CNS), involving the brain and spinal cord to reach the affected cells or neurones. Delivery to the CNS is not straight forward as the medicine must pass through a protective barrier known as the blood brain barrier. The blood brain barrier protects the CNS from harm through infection or invasion from foreign cells or chemicals. To help this version of SRSF1 readily enter cells (and potentially cross the blood brain barrier), the researchers attached a ‘cell permeable peptide’. The ‘cell permeable peptide’ is a small protein which can easily pass through the membrane of cells. The addition of the peptide helps the smaller SRSF1 pass easily into the cells where it would be needed for it to be an effective therapy. For clarity of the terms in this blog the new potential therapy will be known as ‘cell penetrant SRSF1-peptide’ from now on.
This synthesised compound was tested in both cell and animal models of C9orf72 MND including human cells, flies and mice. In all cases, they found that the production of toxic proteins was reduced. In the fly models, they also observed improvements in muscle movement. Overall, the evidence suggests that ‘cell penetrant SRSF1-peptide’ has the potential to be a therapy for people living with C9orf72 MND and FTD.
Could this potential therapy be developed into a nasal spray?
The researchers suggested that, in the future, the SRSF1-peptide could be administered by a nasal spray or other non-invasive methods. The theory being that the ‘cell penetrant SRSF1-peptide’ makes the potential therapy rapidly absorb into cells. However, this is still very much a theory. No research has yet been undertaken to see if it would be possible for the potential therapy to be administered in this way. Further research is needed to see if this is possible and what affect administering by nasal spray may have.
Moving forward
While the results from this pre-clinical work, where it has only been undertaken in a laboratory, is promising. However, these experiments have so far only tested ‘proof of principle’. Further research is now needed to confirm the findings from this research. Only then can testing in humans, through clinical trials, be considered.
This research is another step in the right direction towards uncovering and understanding another complex piece of the MND puzzle. Whilst more research is still needed to move these results towards a potential new therapy, this research provides hope for the future potential treatments for MND. It also provides a scientific foundation to continue to build on and develop. This innovative concept has the potential to transform the development of potential therapies for some other neurodegenerative diseases which are caused by repeat sequences in genes.
How has your support helped drive this research?
The early phase of this work was supported (pump-primed) by a MND Association biomedical grant. Support in the early pre-clinical phases is so important to developing ideas and moving us closer to effective treatments. This work demonstrates how every contribution of each person in our community, past and present, underpins our understanding of MND and moves us closer to effective treatments.
This research and the 104 grants that the MND Association currently supports (as of 31 December 2022) would not be possible without the commitment, dedication and sacrifice of people with MND, their families and carers.