In a study published in Nature Neuroscience this week, a collaboration led by Dr. Jemeen Sreedharan and colleagues from King’s College London, the Babraham Institute and the University of Cambridge have published a new mouse model of Motor Neurone Disease (MND).
The study takes advantage of cutting edge gene editing technology called CRISPR/CAS9 to generate a mouse model of the human disease that accurately mimics a genetic component found in some people affected by MND. The researchers used the gene editing technology to precisely change (mutate) the gene that the body uses to produce the protein TDP-43, a very important player in the MND story implicated in almost all cases of MND.
The power and beauty of the CRIPSR/Cas9 approach is its targeted precision.
The technology which stands to revolutionise every aspect of science may well be one of the most significant breakthroughs of the 21st century. What is incredible in the use of CRISPR/Cas9 is that a single letter of the DNA code can be identified, targeted and changed in this string of 3 billion letters (in human DNA) without affecting any of the others. This is because we are now able to make very small, targeted and precise changes to the genome, with great accuracy without affecting/disrupting any other part of the genome. The technology allows the targeting and precisely planned change of a single letter in the DNA code of the organism. Think of DNA as an instruction manual to build an organism, where the instructions are in the form of a massive string of 3 billion letters similar to a computer code. So there are 3 billion letters in the instructions. CRISPR is able to identify and target a single one specific letter and change it from its original letter to a letter of the researchers choosing.
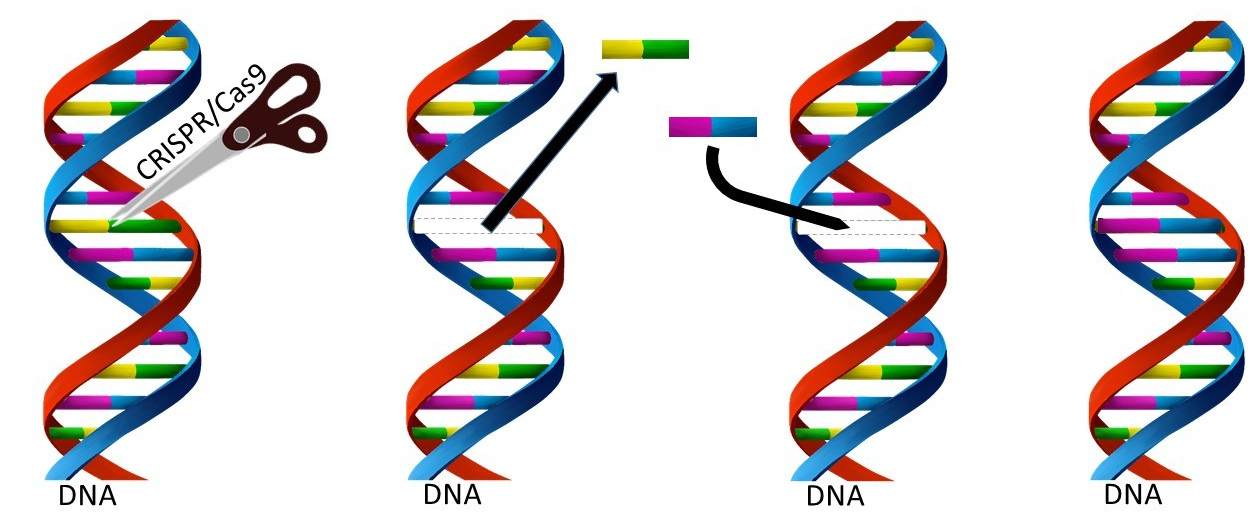
Previously, the process of making animal models incorporating the genetic basis of disease have involved several relatively crude approaches which have not often resulted in a useful model that accurately mimics the human disease. For example, in some previous studies, a whole new gene would be introduced into the genome (either with or without a mutation) or the whole gene would be removed. This ‘sledgehammer’ approach is not what we know occurs in a person with genetic form of MND, where a single mutation may be the basis of disease. The advent of CRISPR/CAS9 gene editing technology has opened exciting new possibilities for the creation of more accurate animal models that will be more relevant by accurately mimicking the genetics of human disease. More relevant animal models offer the possibility to further accelerate the rate of discovery, increasing our understanding of the mechanisms of MND, ultimately bringing us closer to potential treatment and a world free of MND.
The genetic change induced by the team in this study has been shown to occur in people with MND and is highly toxic in many laboratory experiments both in vitro (cells in a Petri dish) and in vivo (in living laboratory animals, including, flies, worms and fish). Dr. Jemeen Sreedharan, the principal investigator, and colleagues introduced the mutation in the exact same position in the gene of the mouse, as it is found in some humans with this genetic cause of MND.
In this mouse, the TDP-43 mutation results in the protein losing its ability to self-regulate, and so the protein levels become higher than normal. This caused a chain reaction that changes to the levels of several other proteins highlighting genes which TDP-43 also normally controls. Interestingly, one of the genes was a gene that encodes “Tau” (rhymes with wow) which is a protein that is associated with Alzheimer’s disease. Perhaps as a result some of the mice had frontotemporal dementia like cognitive changes, just as occurs in around 15% of people affected by MND.
The Marble Burying – Cognitive Assessment
To characterise the symptoms in the mice, specifically cognitive function (see MND- Frontotemporal Dementia (FTD) information below), the group used a system which utilises the natural instinct and behaviour of the mice- marble burying. In the lab the mice live on a deep layer of sawdust. If a marble is placed on the surface of the sawdust the mice will naturally play with and bury the marble. In the test, ten marbles are placed evenly spaced on top of the sawdust, looking rather like the top of a Bourbon biscuit. Usually, 30 minutes later, at least 8 of the 10 marbles will be buried under the sawdust by heathy mice. In contrast, mice with cognitive symptoms associated with Frontotemporal Dementia (FTD) that occurs in some people affected by MND will not bury many of their marbles. This loss of natural burying behaviour has been correlated with cognitive decline and can be used to identify diseased mice from unaffected controls.
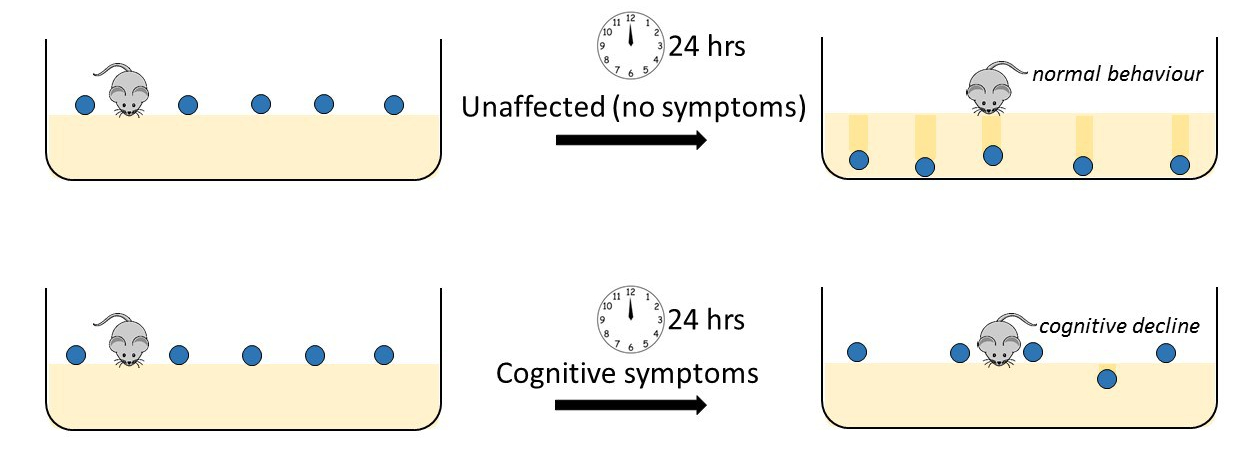
However, it is well known that if you have a mutation in a gene known to cause MND, this does not necessarily mean that you will develop the disease. This has even been shown with identical twins who have the same genes, where one may develop disease and the other never develops symptoms in their lifetime. In addition, the age at which a person will first get symptoms and the severity of the disease can vary considerably between people. It is assumed that some genetic variation and interaction with environment influences this difference in onset and degree of severity of symptoms between individuals.
Just as in humans, only some but not all of the mice in the study with the CRISPR/Cas9 genetic mutation in TDP-43 did show major symptoms. This suggests that some of the mice were resistant to the neurodegeneration whilst others were susceptible. Indicating that other factors within the animals’ biology were at work to protect the unaffected mice from disease. This is a surprising finding since the mice are bred to be as genetically identical as possible and were being kept in the same environmental conditions.
This lack of symptoms in some of mice could have been viewed as a disappointment by the researchers (they were trying to make mice with MND), but in fact it is a good reflection of the range of symptom severity and disease onset seen in the human disease (Just like the identical twins case). The research team turned this to their advantage in order to learn more about the mechanisms that underlie the disease and potential mechanism of neuro protection occurring in the mice.
They split the mice into two groups, those with symptoms and those without, and analysed the genes that were differently activated between the two groups. These mice which do not develop symptoms of disease, just like some people, are perhaps dealt a ‘good hand’ which during their lifetime contains ‘good genes and factors’ that offer protection from the bad ones. By comparing the biology of those mice or people who are resistant to disease with those who show symptoms, they are able to identify modifiers or protective genes. This gives us the opportunity to understand why some people appear to be resistant to disease, or live longer than others.
Identifying affected genes
By comparing the differences in the active genes between the disease resistant mice and those showing disease symptoms the research team identified a number of key changes between the groups. There was a difference in the activity of over 450 genes between the mice without symptoms and those with, that are involved in the mechanism of the disease, and more importantly are associated with protection against symptoms. By identifying these 450 genes, the team are uncovering key targets that offer the potential new avenues for development of therapeutics and further understanding the underlying causes of MND.
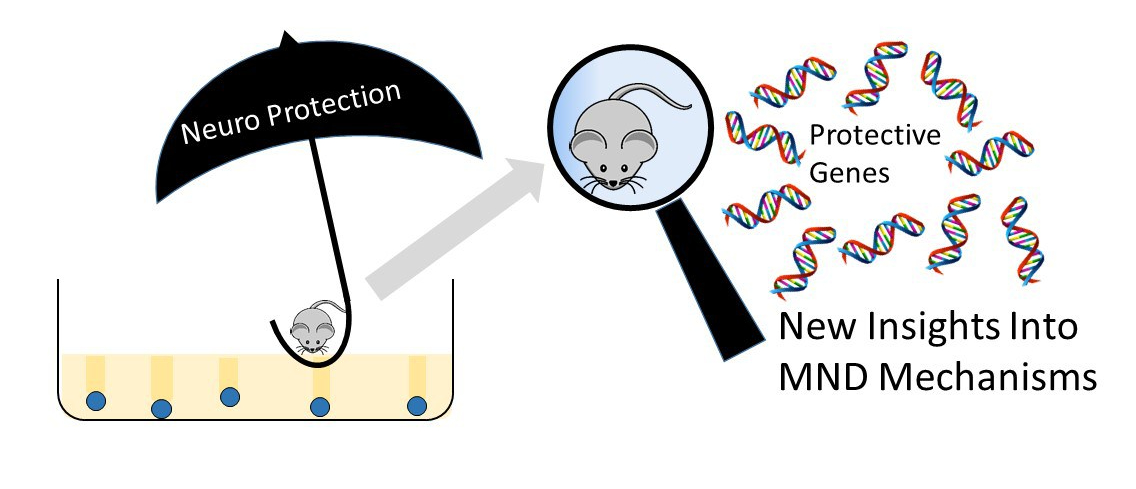
Some of these were genes already known to be important in MND and the health of neurons, providing confidence in the findings. These outcomes provide new avenues in our understanding of the mechanisms at work in both FTD and MND, a disease which currently has no cure or effective treatment, little understanding of the cause and a lifetime risk of 1 in 350. We are delighted to see the outcomes of this MND Association supported work which moves us another step closer to our vision of a world free from MND.
MND-FTD (Motor Neurone Disease-Frontotemporal Dementia).
It is now widely accepted that there is a connection between Motor Neurone Disease (MND) and Frontotemporal Dementia (FTD). FTD is a condition of gradual cognitive change in personality, language and behaviour such as impulsive, compulsive, and emotions behaviour due to loss of function of neurons in the frontal and temporal lobes of the brain. These lobes sit at the forward part of the brain above the eyes and behind the temples. Genetic mutations linked to both MND and frontotemporal dementia (FTD) have been identified. These genetic components predispose people to the condition which is part of a spectrum of disease, ranging from pure ALS or pure FTD at either end of the spectrum and ALS combined with FTD to varying degrees in between.
Listen to Dr. Jemeen Sreedharan talk about this work here in a Naked Scientist Special Podcast (7mins)





